Details of the Drug
General Information of Drug (ID: DMQYV60)
| Drug Name |
Isopropamide iodide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Isopropamide Iodide; 71-81-8; Darbid; Isoproponum iodide; Priamide Eupharma; Priamide; Tyrimide; Dipramide; Priazimide; Piaccamide; Sanulcin; Dipramid; Isamid; Marygin-M; UNII-E0KNA372SZ; Isopropamide ioduro [DCIT]; Isopropamidi iodidum [INN-Latin]; SKF 4740; Iodure d'isopropamide [INN-French]; EINECS 200-766-8; NSC 15521; Ioduro de isopropamida [INN-Spanish]; 5579 MD; E0KNA372SZ; R 79; MLS000069749; (3-Carbamoyl-3,3-diphenylpropyl)diisopropylmethylammonium iodide; Benzenepropanaminium, gamma-(aminocarbonyl)-N-methyl-N,N-bis(1-methyl
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
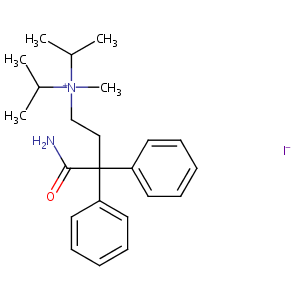 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 480.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
